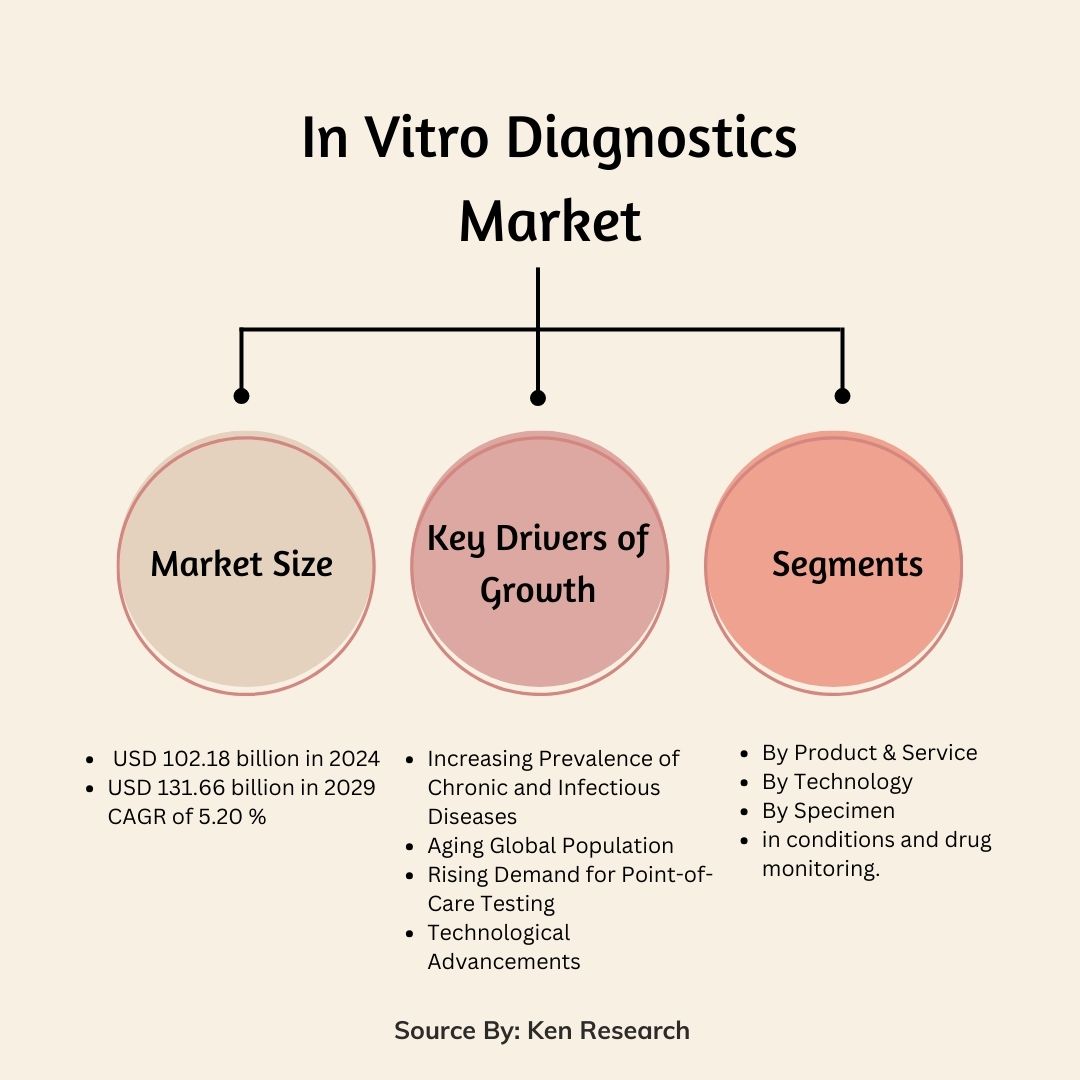
As of 2024, the In Vitro Diagnostics industry has reached an impressive value of USD 102.18 billion. The market is not just sizable but is also on a rapid growth trajectory. Projections indicate that by 2029, it could soar to USD 131.66 billion, reflecting a compound annual growth rate (CAGR) of 5.20%. This article explores the primary drivers, key segments, trends, and opportunities that are shaping this dynamic market.
Key Drivers of Growth in the In Vitro Diagnostics Market
Several factors are contributing to the robust expansion of the in vitro diagnostics market
- Increasing Prevalence of Chronic and Infectious Diseases: Chronic conditions like diabetes, cancer, and cardiovascular diseases are becoming more widespread globally. These ailments often require continuous monitoring, driving the demand for IVD products. Additionally, the ongoing threat of infectious diseases, such as COVID-19, has further underscored the importance of rapid and accurate diagnostics.
- Aging Global Population: With a significant rise in the elderly population worldwide, the demand for diagnostic tests market related to age-associated diseases is increasing. The growing geriatric demographic is particularly susceptible to chronic illnesses, which necessitates regular diagnostic testing.
- Rising Demand for Point-of-Care Testing: Point-of-care (POC) testing offers the advantage of delivering quick diagnostic results at the location of patient care, bypassing the need for traditional lab tests. This is especially beneficial in emergency situations and in areas with limited access to full-scale diagnostic facilities.
- Technological Advancements: The IVD industry is at the forefront of technological innovation. New developments are leading to more precise, faster diagnostic tests, with advancements in molecular diagnostics and the miniaturization of instruments for POC testing playing key roles.
- Emphasis on Personalized Medicine: Personalized medicine, which customizes treatment based on individual genetic profiles, relies heavily on IVD tools. These diagnostics provide essential insights that guide tailored therapeutic decisions, making them integral to the advancement of personalized healthcare.
Segments of the In Vitro Diagnostics Market
The In Vitro Diagnostics industry is categorized across various segments to better understand its growth trends and target specific areas:
By Product & Service
- Instruments: Devices used for conducting diagnostic tests.
- Kits & Reagents: Consumable products like test strips and antibodies that are essential for testing.
- Software: Tools for analyzing and interpreting diagnostic data.
By Technology
- Immunoassay: Detects specific antigens or antibodies in a sample.
- Molecular Diagnostics: Examines genetic material to identify pathogens or genetic anomalies.
- Hematology: Analyzes blood cells for diagnosing blood-related conditions.
- Urinalysis: Tests urine samples to identify potential health issues.
By Specimen
- Blood, Serum & Plasma: Common specimens offering broad diagnostic insights.
- Urine: A non-invasive method for health screening.
- Saliva: Increasingly used for certain conditions and drug monitoring.
By Application
- Infectious Diseases: Vital for diagnosing diseases like COVID-19, HIV, and flu.
- Oncology: Used in cancer screening, diagnosis, and monitoring.
- Autoimmune Diseases: Helps diagnose conditions such as lupus and rheumatoid arthritis.
- Cardiovascular Diseases: Assesses the risk of heart conditions.
- Diabetes: Monitors and diagnoses diabetes.
Current Trends in the In Vitro Diagnostics Industry
The IVD market is witnessing several key trends that are shaping its future:
- Growth in Point-of-Care Testing: POC diagnostics are gaining popularity due to their convenience and speed. This trend is expected to continue, driven by the development of more advanced testing technologies.
- Automation and System Integration: Laboratories are increasingly adopting automation to boost efficiency and reduce errors. There is also a growing focus on integrating IVD instruments with data management systems for smoother workflows.
- Expansion of Telehealth and Remote Diagnostics: The integration of IVD with telehealth platforms is opening up new possibilities for remote diagnostics, particularly benefiting patients in remote areas or with limited mobility.
- Advances in Molecular Diagnostics and Personalized Medicine: Molecular diagnostics are becoming crucial for identifying genetic variations, enabling personalized treatment approaches. IVD companies are innovating in this space to support the growing demand for personalized healthcare.
- Shift Toward Value-Based Care: The healthcare industry is moving towards value-based care, where outcomes are prioritized over service volume. IVD tests that provide cost-effective and clinically significant results are becoming more attractive in this environment.
- Data Analytics and AI Integration: The incorporation of AI and data analytics in IVD is on the rise, leading to improved diagnostic accuracy, faster decision-making, and the development of predictive tools.
- Evolving Regulatory Landscape: The regulatory environment for IVD products is continuously changing, requiring companies to stay updated to ensure compliance and smooth product launches.
Challenges and Opportunities in the In Vitro Diagnostics Sector
While the IVD market is poised for significant growth, it also faces challenges:
Challenges
- Budgetary Constraints: Tight healthcare budgets can limit the adoption of expensive IVD technologies. Striking a balance between cost-effectiveness and clinical benefits is critical.
- Regulatory Hurdles: The stringent regulations governing the IVD market can slow down innovation and delay product launches.
- Cybersecurity Concerns: As IVD systems become more digital, protecting patient data from cyber threats becomes increasingly important.
- Reimbursement Issues: Inadequate reimbursement policies can hinder the adoption of IVD tests, affecting their accessibility.
Opportunities
- Emerging Markets: Growing economies in regions like Asia-Pacific and Latin America present vast opportunities for the IVD industry, driven by rising healthcare investments and demand for advanced diagnostics.
- Telehealth and Home Healthcare: The growing trend of telehealth and home-based care creates new avenues for remote diagnostics and point-of-care testing.
- Value-Based Care Models: IVD tests that contribute to improved patient outcomes at lower costs are well-positioned to thrive in a value-based care environment.
- Mergers and Acquisitions: Strategic mergers and acquisitions can accelerate innovation and expand market reach, providing comprehensive diagnostic solutions.
- Artificial Intelligence: AI integration in IVD is set to revolutionize diagnostics by enhancing data analysis, improving diagnostic accuracy, and supporting personalized medicine.
Regional Insights
- North America: This region holds a substantial share of the IVD market, driven by a well-established healthcare infrastructure and a high incidence of chronic diseases, particularly in the United States.
- Europe: With strong markets in Germany, France, and the UK, Europe remains a key player in the IVD sector, supported by innovation in healthcare and strong regulatory frameworks.
- Asia-Pacific: Rapid growth in healthcare investments and infrastructure, coupled with a rising prevalence of chronic diseases, is propelling the IVD market in Asia-Pacific.
- Middle East and Africa: The adoption of IVD technologies in this region is growing as healthcare systems improve and awareness of diagnostic importance increases.
- South America: Countries like Brazil and Argentina are driving the expansion of the IVD market, supported by a focus on healthcare improvements and the rising incidence of chronic diseases.
Read Also:- India’s $1.7 Billion Rapid IVD Kits Market Size, Growth And Future Outlook
Conclusion
The In Vitro Diagnostics market is a rapidly evolving and essential component of modern healthcare. Driven by technological advancements, the increasing prevalence of chronic and infectious diseases, and a shift towards personalized medicine, the market is poised for significant growth in the coming years. Continued innovation and strategic collaborations will be key to unlocking the full potential of this dynamic sector, leading to improved diagnostics and better healthcare outcomes.



